Integrating multi-omics data into predictive models has the potential to enhance accuracy, which is essential for precision medicine. In this study, we developed interpretable predictive models for multi-omics data by employing neural networks informed by prior biological knowledge, referred to as visible networks. These neural networks offer insights into the decision-making process and can unveil novel perspectives on the underlying biological mechanisms associated with traits and complex diseases. We tested the performance, interpretability and generalizability for inferring smoking status, subject age and LDL levels using genome-wide RNA expression and CpG methylation data from the blood of the BIOS consortium (four population cohorts, Ntotal = 2940). In a cohort-wise cross-validation setting, the consistency of the diagnostic performance and interpretation was assessed. Performance was consistently high for predicting smoking status with an overall mean AUC of 0.95 (95% CI: 0.90–1.00) and interpretation revealed the involvement of well-replicated genes such as AHRR, GPR15 and LRRN3. LDL-level predictions were only generalized in a single cohort with an R 2 of 0.07 (95% CI: 0.05–0.08). Age was inferred with a mean error of 5.16 (95% CI: 3.97–6.35) years with the genes COL11A2, AFAP1, OTUD7A, PTPRN2, ADARB2 and CD34 consistently predictive. For both regression tasks, we found that using multi-omics networks improved performance, stability and generalizability compared to interpretable single omic networks. We believe that visible neural networks have great potential for multi-omics analysis; they combine multi-omic data elegantly, are interpretable, and generalize well to data from different cohorts.

Over the last decades, association studies have uncovered numerous genes and CpGs to be associated with hundreds of traits and diseases 1 . This has led to tools for identifying high-risk individuals and biomarkers for early disease detection. For example, blood-based methylation biomarkers are currently used for early diagnosis of various forms of cancer 2,3 . However, for most complex diseases and traits, the combined effects, within and between different omics types, are still largely unexplored. For a more comprehensive understanding of human health and diseases and for more accurate prediction models, it is therefore necessary to study omic types in relation to one another. Thanks to recent technological improvements for high throughput sequencing and arrays technologies, the acquisition of multi-omics datasets has become more feasible, providing opportunities for new multi-omics analysis tools 4,5 .
Recently, novel statistical frameworks and machine learning techniques have been published that integrate multi-omics data in a single analysis 6,7 . These studies show the potential for multi-omics analysis to improve prediction for various disorders while providing insight into disease biology 4,8 . Integrating different types of omics data in a single analysis is a challenging task, as each type has different, procedures, preprocessing steps and analytical requirements 9 . Combining omics data presents additional challenges, as each omic has unique dimensions, and it is essential to consider correlation structures both within and between the different omics types. Thus, for the combined analysis of multiple omics types, methods need to be flexible and be able to deal with the high dimensionality of these datasets.
Neural networks have demonstrated such flexibility and have been widely successful in fields such as image classification 10 , speech recognition 11 , and protein modelling 12 . In contrast to most tasks in image analysis and speech recognition, the focus of multi-omics frameworks is not only on predictive performance but also on understanding the underlying aetiology. To facilitate this, a new field in machine learning, coined visible machine learning 13 emerged, in which prior biological knowledge is embedded in a neural network’s architecture to create interpretable neural networks 14,15,16,17,18,19 . Recent examples of these kinds of neural networks applied in genomics are GenNet 20 and P-net 21 . In the GenNet framework, gene and pathway annotations were used to create interpretable neural networks for genetic risk prediction from genotypes. In P-net, methylation, gene expression and copy number variants were fed to an interpretable neural network to differentiate between primary or metastatic prostate cancers. Importantly, recent work evaluating the reliability of the interpretations of P-net found that the interpretations can be strongly affected by different weight initializations 19 . Other examples of visible neural networks include, PasNet 22 , which integrated pathways information to predict survival for glioblastoma multiforme, a primary brain cancer. DrugCell 23 integrated Gene Ontology knowledge in a network to predict drug response for various cancers and ParsVNN 24 continued on this work and pruned the network for increased performance and better interpretability.
In this study, we employ visible neural networks to analyse multi-omics data. We extend the GenNet framework to create interpretable neural networks for multiple omics inputs and apply it to a dataset with transcriptomics and methylomics data. We validate the method using four cohorts in the BIOS consortium for the application of predicting age, low-density lipoproteins (LDL) levels and smoking status. Age prediction from methylation or gene expression data has been an active research area popularized by the work of Hannum et al. and Horvath 25,26 . Additionally, it has been shown that these clocks show an asymptote for older participants and strong biological sex differences, making age prediction particularly interesting to study with neural networks 27 . Smoking status and LDL level predictions are well-suited to evaluate the performance, stability and interpretation of the method. Methylation and gene expression are highly predictive for smoking status and predictive genes are well-documented 28,29 . On the other hand, low lipid lipoprotein cholesterol levels are a complex outcome with both environmental and genetic factors 30 .
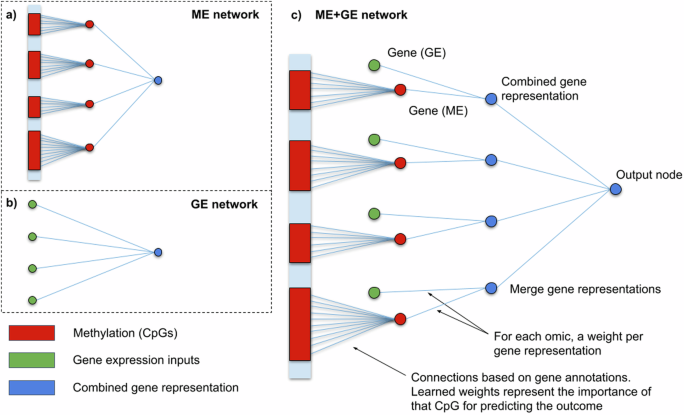
We present the following contributions to this work. First, we have extended GenNet to create visible neural networks that can predict outcomes from multi-omics data while quantifying the importance of each omic type, gene, and pathway. In the proposed network, we merge each omic at the gene level, enabling gene-wise extraction of the importance of each omic for prediction. Secondly, we investigate the robustness of the performance and interpretations of these visible neural networks across three different phenotypes. By leveraging the multi-cohort setting of the BIOS consortium in cohort-wise cross-validation, we can examine the stability of these networks across different random seeds and cohorts. Finally, we perform additional analyses to identify omic-specific information, gene-covariate interactions, and group-specific patterns learned by the visible neural networks.
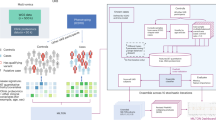
In this study, multi-omics data gathered by the Biobank-based Integrative Omics Study (BIOS) consortium was used to predict smoking status, age and low-density lipoprotein levels. Specifically, we used transcriptome and methylome data from BIOS's four largest cohorts: LifeLines (LL), Leiden Longevity Study (LLS), Netherlands Twin Register (NTR), and Rotterdam Study (RS) to evaluate the performance and the interpretations of the created neural networks in a cohort-wise cross-validation. Importantly, all cohorts within the BIOS consortium followed the same procedure in gathering and processing data (see “Methods”). An overview of the characteristics of each cohort can be found in Table 1.
The activation function transforms the output signal for each neuron. For classification tasks, such as predicting if an individual smokes or not, a sigmoid activation function was used to scale the output to the range [0, 1] in the last neuron. Arctanh activation functions were used for all other layers to introduce non-linearities, increasing the modelling capabilities of the network. For regression tasks, such as predicting continuous traits such as age and LDL levels, ReLu activation functions with output range [0, ∞) were used for all layers. For a better initialization of the network, the bias of the last neuron was set to the mean value of the predicted outcome in the training set.
For more complex modelling of the interactions between expression, methylation and phenotypes, we also evaluated deeper neural networks. First, using KEGG’s functional pathways 32,33 as prior knowledge, three hierarchical pathway layers were created (Fig. 2b). The first layer groups genes into 321 functional pathways such as: insulin secretion, thyroid hormone synthesis and PPAR signalling pathway. The aforementioned pathways are all part of the endocrine system group which, in turn, is a subgroup of organismal systems. The mid and global-level pathway layers were created adopting this hierarchical structure, consisting of 44 and 6 groups, respectively. Each pathway is represented by its own neuron resulting in three layers with 321, 44 and 6 nodes each. Not all genes were annotated by the KEGG functional pathway annotations, 5591 genes did not receive a functional pathway annotation. To ensure connectivity to the output for all genes, connections that skip the pathway layers (skip connections) were added from each gene to the output node (see Supplementary Fig. 2 for the distribution).
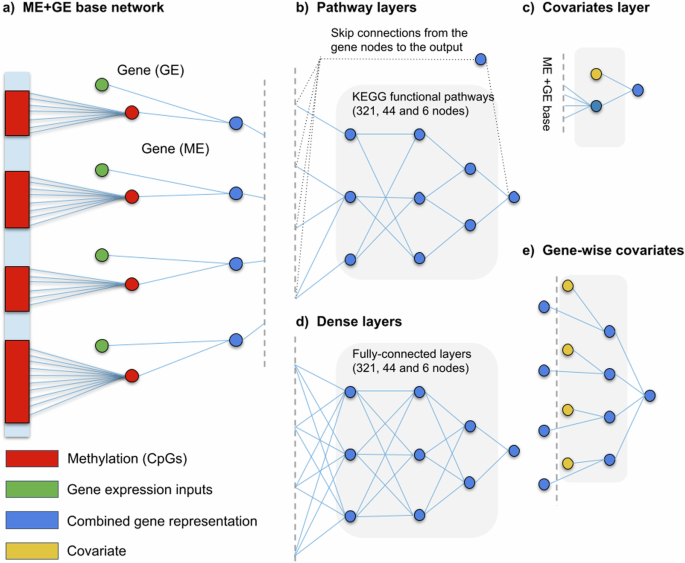
Additionally, a deeper network was constructed without any additional prior biological knowledge to compare with the KEGG pathway network (Fig. 2d). Similarly, the ME + GE network served as a basis for this network and three densely connected layers, 321, 44 and 6 nodes each, were added between the gene layer and the output node. The resulting network has thus the same number of neurons as the KEGG pathway network but has fully connected layers instead of layers based on KEGG pathway information.
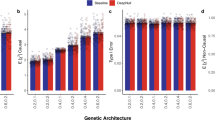
An overview of the performance for each cohort and for the three different architectures can be found in Table 2. It shows the mean predictive performance and standard deviation for each fold for ten networks trained with the same hyperparameters but with different random seeds. The corresponding hyperparameters, chosen on the best performance in the validation set, can be found in Supplementary Table 3.
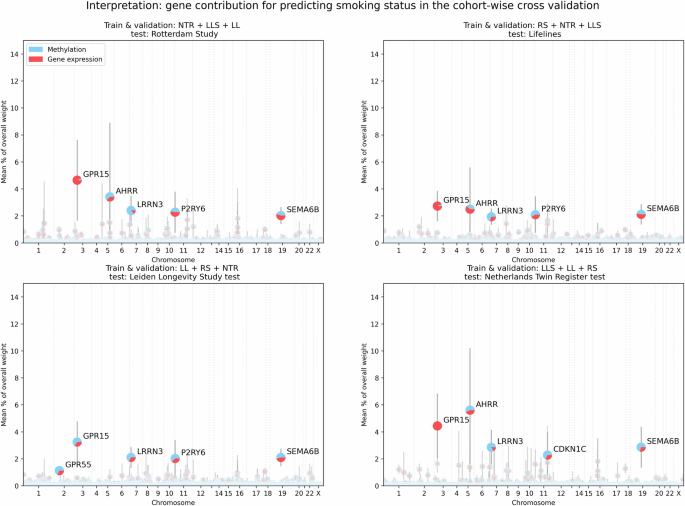
To investigate the interplay between the omic types and to find omic-specific information, two additional analyses were conducted where either gene expression or methylation gene representations were penalized (see Supplementary Figs. 4–6). Without penalization, the weights for gene expression and methylation were nearly equally divided after training. Weights connected to gene expression input occupied 51.6 ± 1.3% of the weights over all the ME + GE networks, with the remainder used for methylation. In these experiments, we found that an omic-specific L1 penalty of 0.01 for gene expression reduced the contribution of the weights associated with gene expression to 0.69 ± 1.16% while a similar threshold reduced the weights associated with methylation to 2.56 ± 1.73%. A more severe omic-specific L1 threshold of 0.001 for methylation reduced the use of methylation in the top genes nearly completely, only for LRNN3 methylation input is still used in the second and third fold with (respectively ~41% and 16% of the weights for this gene). However, with the same threshold gene expression inputs are responsible for 15% of the weights for AHRR in the first fold, nearly 29% of the GPR15 weights in the second fold and 39% of RER1 in the fourth fold (see Supplementary Figs. 3 and 4). Interestingly, the importance of AHRR was severely impacted by the methylation penalty, its gene expression was barely used to predict smoking status when methylation was penalized.
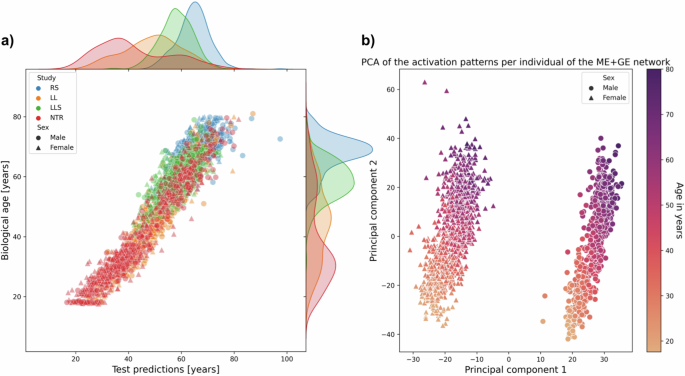
Networks trained with both methylation and gene expression data (ME + GE) achieved a mean error of 5.16 (95% CI: 3.97–6.35) years overall folds for age prediction (see Table 2). Between folds, there were large differences in performance for predicting age. Most notably, networks did not generalize well in folds that have either the RS (ranging between 52 years and 80 years) or the LLS (ranging between 30 years and 79 years) as test cohorts, the two cohorts with the oldest population. For these cohorts, the explained variance in the test set was substantially lower than in the validation set: RS test 0.40 (95% CI: 0.37–0.43), 0.94 (95% CI: 0.93–0.94) validation, LLS test 0.95 (95% CI: 0.95–0.95), 0.61 (95% CI: 0.60–0.63) validation. Aside from being older, these cohorts also have a smaller spread in age distribution compared to the two other cohorts (See Fig. 4a and Supplementary Fig. 7). The NTR cohort ranges between roughly 18-years-old and 80-years-old while individuals from the LL cohort were between 18-years-old and 81-years-old.
Differences between omics and network types were also larger for age prediction than for smoking status prediction. The ME + GE network consistently outperformed the single-omic networks with substantial margins: the mean explained variance over all folds was 0.72 (95% CI: 0.36–1.07) for the ME + GE network, 0.30 (95% CI: −0.26 to 0.86) for gene expression, while the ME networks did not find any predictive pattern that translated to the test cohort. Training and validation performance was generally poor for the ME network, and although the GE network obtained good validation performance in terms of explained variance for each fold, this did not translate in folds with the RS and LLS as test cohorts. The baseline network performed substantially better than the visible neural network for the methylation data with an error of 4.10 years (95% CI: 2.73–5.46) versus that of 15.00 (95% CI: 4.31–25.69) for the ME network. However, the performance of the ME + GE network was slightly better (root mean squared error (RMSE) of 5.16 years, 95% CI: 3.97–6.35) than the multi-omic baseline network (RMSE of 5.90 years, 95% CI: 4.59–7.21).
Interpretation of the ME + GE network revealed that many genes had a small contribution to age prediction (see Supplementary Fig. 7). The neural network found a more multifactorial solution for age prediction than for smoking, the most important gene over all folds only occupied 0.68% of all weights for predicting age compared to 3.76% for smoking. The most predictive genes with a weight contribution higher than 0.30% of the total weight in three out of the four folds were COL11A2, AFAP1, OTUD7A, PTPRN2, ADARB2 and CD34 (Supplementary Table 6). These most predictive genes were not part of Hannum et al. and Horvath’s epigenetic clocks 25,26 . The first principal components of the activation patterns of the ME + GE network revealed distinct activation patterns for the different sexes with a gradient in each cluster (see Fig. 4b). However, there is no significant difference in the absolute error between the sexes (Wilcoxon rank-sum, p-value of 0.98, Supplementary Figs. 8 and 9), the first principal component clusters perfectly for males and females while the second principal component is strongly related with age. Additional experiments showed that the clustering of the sexes is mainly driven by genes on the X chromosome (see Supplementary Fig. 10). Including sex as a covariate in the last layer of the model did not improve the performance of the model (mean RMSE overall folds of 7.31 [95% CI: 2.89–11.73]). Including sex information to each gene also did not lead to a better performance (mean RMSE overall folds of 10.64 [95% CI: 4.12–17.15]). However, inspecting the weights between the covariate and the genes for the best-performing network revealed strong sex-specific weights for, among others: KLF13, ANO9 and HECA (for more details see Supplementary Fig. 11). For these genes the network needed strong weights to model sex-specific effects for age prediction.
After applying an omic-specific L1 penalty for methylation of 0.01, the network only used the methylation input for gene NEDD1 in the second fold with nearly 33% of the weight contribution for this gene from methylation, while in the third fold MAD1L1 had a methylation contribution of 23% (see Supplementary Fig. 14). With the same threshold for penalizing gene expression inputs, DNAJB6 had the largest gene expression use with 31% of the weight for this gene assigned to gene expression input (Supplementary Fig. 15). The deeper neural network architectures quickly overfitted, reaching high performance on the training data which did not generalize to the validation and test set. These networks were consistently outperformed by the ME + GE network (Supplementary Table 7). The best-performing network built with KEGG pathway information had the pathway: “environmental information processing” as the most predictive global pathway because of the high contributions of membrane transport (ABC transporters), signal transduction, and signalling molecules and interaction (see Supplementary Fig. 16).
ME + GE and GE networks explained up to 17% of the phenotypic variance in the validation set but these networks only generalized in the second fold to an explained variance of 0.07 (95% CI: 0.05–0.08) for the ME + GE network and 0.04 (95% CI: 0.04–0.05) for the GE network in the LL test cohort (see Supplementary Table 8). In this fold, the largest gene, FAM53A only occupied 0.052% of the total weight (Supplementary Fig. 17). The weights for all genes in the ME + GE network were small and evenly spread, indicating that the network did not find individual genes with a strong effect for predicting LDL levels. Additional layers, be it pathways or densely connected layers, did not improve predictive performance.
In this paper, we evaluated the performance, interpretability and stability of visible neural networks for single and multi-omics data. Interpretability was achieved by embedding prior biological knowledge such as gene and pathway annotations in the neural network architecture. We applied these models to predict smoking status, age and low-density lipoprotein levels in a cohort-wise cross-validation using methylation and gene expression data. For smoking, single omic networks and multi-omic networks performed consistently high across all cohorts for predicting smoking status. Predicting smoking status is a relatively simple task, since smoking is a powerful inducer of DNA methylation and gene expression alterations 37 . This is also reflected by the mean AUC of 0.95 overall folds that the ME + GE and ME networks achieved. It is slightly better than the performance of Maas et al. who reported an AUC of 0.90 in an external dataset with a weighted combination of just thirteen CpGs. Inspection of the weights of the ME + GE network revealed GPR15, AHRR and LRRN3 as the most important genes for prediction, which is consistent with existing literature 28,29,37,38 . In the ME + GE network, the contribution of both omics types was nearly equal (in terms of weights), while the gene expression-based network by itself was less predictive than the methylation-based networks. Applying an omic-specific penalty for methylation input showed that the ME + GE network needed some methylation input to achieve similar performance with expression information.
For predicting age, the ME + GE network outperformed the single ME or GE networks. The performance of this network in the test cohorts varied between an R 2 of 0.40 (95% CI: 0.37–0.43) and 0.91 (95% CI: 0.90–0.92). This difference in performance is probably caused by the different distributions in age in the cohorts, depending on the cohorts in the training set the networks are shown fewer examples of older or younger individuals. Similar effects were also seen in traditional methods 9 . Based on the predictive performance shown in Table 2 one could conclude that for age prediction, usage of the two omics types increased stability and performance for these types of neural networks compared to the single omic networks. Additionally, we have evaluated whether the network used sex information in the decision process for age prediction. The first principal component of the activations of the neural network showed a perfect separation between the sexes, mostly caused by genes on the X chromosome, while the second principal component had a clear correlation with age. Owing to the shallowness of the networks, the activation pattern will therefore closely resemble the underlying data, especially if it has some relation with the outcome. For deeper networks, a PCA on the activation may reveal more detailed information (such as different patient subtypes or mediating factors) since the network applies more complex transformations to the data. The inclusion of genes on the X-chromosome allowed the network thus to separate between the sexes but it did not have the capacity to model different effects independently from the input for each sex. To help the model to find a sex-specific effect we modified the network with sex information as an extra input to each gene node. After, training the network found the strongest sex-specific gene effects for KL13, ANO9 and HECA. However, this addition to the network architecture did not improve performance.
An earlier EWAS in only the RS did not find significant associations between DNA methylation in blood and LDL cholesterol 39 . Another EWAS using BIOS data found only three significant associations, demonstrating that there is a very weak relation between methylation and LDL measurements from blood which makes the prediction task more complex 40 . The neural networks did find patterns in the training set that were also found in the validation set (up to an R 2 of 0.17 [95% CI: 0.16–0.18]) but this pattern did not generalize to the test cohorts with the exception of the Lifeline cohort. In this cohort the method achieved an R 2 of 0.07 (95% CI: 0.05–0.08) in the test set, substantially lower than the performance of the validation set 0.13 (95% CI: 0.12–0.14). suggesting that the model had trouble generalizing to data from an unseen cohort. Overall, the low prediction performance might also indicate that the studied omic data (gene expression and methylation from blood) might not contain enough information to accurately predict LDL levels.
In general, we found that including multiple omics inputs in the network improved performance. These multi-omic networks had a more stable performance and generalized better to the test cohorts. Surprisingly, deeper networks did not lead to better performance. Generally, one would expect deeper networks to perform better since they can model more complex interactions. Thus, it is possible that the optimal hyperparameter values for deeper networks lie outside the considered hyperparameter range or that more training examples are required to train these deeper networks. Interpreting the ME + GE networks revealed well-known genes such as GPR15 and AHRR for smoking that validate the results. However, we also saw that the interpretation can vary between different random initializations, and it is therefore recommended to train networks with different random seeds for a more complete overview of important predictors. As for all prediction models, it is important to consider that predictive genes and pathways found are not necessarily causal genes and pathways as effects can be mediated. However, these genes and pathways do provide insight into the decision process of the neural network and may be used in follow-up.
For good interpretation, proper regularization is important as it forces the network to use the most predictive input features. For example, an L1 penalty on the weights will force the network to learn sparse weights, resulting in a less complex model. In the absence of an L1 penalty on the weights, the network has more freedom to choose its weights. This does not necessarily harm performance but may harm interpretability. In this work, we use the L1 penalty to regularize the network, but other regularization methods could have been chosen. For example dropout 41 , this method drives the network to find a more stable solution by deactivating random sets of neurons during training. Another important factor for interpretation in visible neural networks is the quality of the prior knowledge used in creation. In this study, the annotations for the CpG sites were based on genomic distance. Potential improvements could come from using tissue-specific and functional annotation databases such as ENCODE 42 .
We believe that visible neural networks have great potential for genomic applications, especially for multi-omics integration. These interpretable neural networks can combine multi-omics data elegantly in a single prediction model and provide the importance of each gene, pathway and omic input for prediction. Additionally, we found that using multi-omic networks generally improved performance, stability and generalizability compared to interpretable single omic networks.
Transcriptome and methylome data from the four largest cohorts in the Biobank-based Integrative Omics Study (BIOS) consortium—LL, LLS, NTR and RS—were used to train the models in a cohort-wise cross-validation setting. In the BIOS consortium, all cohorts adhered to the same procedure in gathering and processing data. For each participant, the transcriptome and the methylome were measured in whole blood samples taken from the same visit. DNA methylation was profiled according to the manufacturer’s protocol using the Infinium Illumina HumanMethylation 450 k arrays, while blood was first depleted from globin transcripts for RNA sequencing. A detailed description of all data generation and preprocessing steps for the RNA sequencing and DNA methylation data can be found in refs. 43,44 . Using the BBMRI-NL’s Integrative Omics analysis platform 45 , all individuals that had both RNA-seq and methylation data (β-value) available were selected, resulting in a dataset with 2940 individuals. Y-chromosomal data was excluded, and X-chromosomal and autosomal measurements were included. Finally, RNA-seq expression data was filtered using an expression inclusion criterion of one count per million on average across all samples or higher 9 .
The neural networks were evaluated in a cohort-wise cross-validation setup (Supplementary Fig. 1) to assess the generalizability of the models across cohorts. In each fold, one cohort is held out as a test set, while the three other cohorts were used for training and validation (leave-one-out method). From these three cohorts, 75% of the individuals were randomly selected for the training set while the remaining 25% was used in the validation set to tune the hyperparameters. For all methods, the same combinations of hyperparameters were tuned on the validation set. Combinations included learning rates of [0.01, 0.001, 0.005, 0.0001] and L1 penalty on the weights of the combined gene and/or methylation gene layer of [0.01, 0.001, 0.0001]. A higher L1 penalty increases the cost for the network to include more contributors to predict the outcome (see Supplementary Note 1). The L1 penalty thus enforces sparsity over the weights, so that most inputs get assigned a (near) zero weight while important inputs still get assigned a high weight. This L1 regularization on the weights helps prevent overfitting and increases interpretability.
The mean squared error (MSE) was used as a loss function to optimize for regression tasks. For classification tasks, weighted binary cross-entropy, with a weighted inverse to the ratio of the class imbalance, was used as a loss function. The loss function quantifies the difference between the current outcome and the true label and is optimized during training. The performance of the resulting network is evaluated using the AUC for classification tasks, and the RMSE and explained variance (sci-kit learn, explained_variance_score) for regression tasks. For each fold the hyperparameters of the best-performing model in the validation set were selected to evaluate the test cohort. Since neural networks use stochastic processes that can influence the outcome, we trained the network with the best hyperparameters ten times with a different random seed to investigate its stability.
The contribution scores for interpreting the importance of each input and node are calculated using the absolute weights in the network. For each path between the output and the inputs, the product of all absolute weights along the path is computed. The total contribution of an input is retrieved by summing the products of all paths that converge on it. The proportion of each input’s contribution relative to the total contribution of all inputs is the final score. To determine the contribution of nodes in hidden (intermediate) layers, this proportion is multiplied by all the weights along the path from the input to the hidden node. Again, the final contribution percentage is calculated as the contribution of the hidden node divided by the total contribution of all hidden nodes in the same layer.
To compare the performance of visible neural networks to more traditional neural networks a baseline network with a locally connected layer followed by two dense layers was trained (see Supplementary Note 2). The locally connected 1D layer has been successfully used in various applications in genomics 46,47,48 and can be seen as a hybrid between convolutional and fully connected layers 49 . For the multi-omics data, the methylation and gene expression data were first concatenated before feeding to the network. Hyperparameters, loss functions and activation functions were optimized and configured in the same manner as for the visible neural networks.
Neural networks are flexible methods and with the inclusion of prior biological knowledge different architectures can be explored to provide more insight into the interaction between omics types, the contribution of covariates and the gene-specific contribution of covariates. For each of these analyses, we made small changes to the ME + GE networks.
Gene expression and methylation data contain redundant information with respect to each other. However, not all information that is present in the one may be present in the other data type. To evaluate the independent contribution of each omics to the prediction we add a L1 penalty for one omics type in the model. This introduces a trade-off for the neural network: the gain in performance for including information on the penalized omic (i.e. RNA expression of a single gene or the methylation representation of a single gene) must outweigh its penalty. If the model uses only the non-penalized omic type without loss of prediction performance, it is likely that there was no omic-specific information. However, if the model still decides to use parts of the penalized omics data, this information is most likely unique to the penalized omic type and was therefore required for prediction.
Including covariates in the model, for example, sex and age for smoking can improve performance and interpretation. Commonly, the covariates are included as an extra layer at the end (Fig. 2c). However, by adding a covariate for each gene, more specific information on how a covariate affects a single gene can be obtained (see Fig. 2e). For each phenotype we tested both, a model with covariates in the last layer and a model with covariates for each gene.
In contrast to fully connected neural networks, the visible neural network architectures used in this study are constructed based on prior biological knowledge that can be interpreted by inspecting the weights of the incoming and outgoing connections. The strength of the weights (e.g. between CpGs and genes, expression and genes, genes and pathways), all express the importance of these biological elements for the predicted outcome. The weights of a neural network are a result of an optimization over the population it was trained on and are thus a result of the population characteristics of the training set. However, neural networks may learn different patterns for the same outcome. By inspecting the weights general information is learned about the importance of each element but this does not show differences between groups or individuals. Based on differences between individuals, some neurons can activate for a certain group of individuals, while others do not for others. To gain an overview of the different patterns that are learned by the network we applied principal component analysis 50 (PCA) over all the activations for all (gene-level) nodes for each individual (see Supplementary Note 3). In this PCA, individual-level differences may cluster and provide groups of individuals for which the neural network used a similar activation pattern.